As of lately I'm reading more and more post where people ask "can I loss fat with just test only"? - "Will TRT help me loss weight"?
The answer is YES, but you have to put in your dedication with nutrition and the appropriate training regiment for YOU!
Below is some useful information that will help assist and broaden your understanding of how TRT (Testosterone will assist with the fat burning process)
------------------------------------------------------
The truth about fat loss and testosterone (got fat, pass the Testosterone)
You Don't need multi compounds... Test alone with diet in check will yield exceptional results!
Below This study we have provided one of the most underrated and pronounced Test esters ever created and used in clinical/therapeutic therapies,It's very diverse, serving multi purposes!
There's been some discussions lately in regards to Testosterone and fat loss..Or Testosterone only cycles vs people advocating BF% must be low before starting a FIRST cycle, or fat loss cycle ext..
I've stressed from time and time again about the bro-science behind "Lower your body fat before your first cycle"..
Below is some illustrations compiled of reads/studies that I have gathered behind the benefits of testosterone and fat loss..
Team Supervisor PSL
Vision
______________________________________-
The first illustration is a study conducted with TRT
Testosterone therapy in hypogonadal men results in sustained and clinically meaningful weight loss.
Abstract
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT:
Hypogonadism is associated with increased fat mass and reduced muscle mass, which contributes to obesity and health risks, such as cardiovascular disease.Testosterone treatment of hypogonadal men improves muscle mass and reduces fat mass; however, many of these studies are of short duration.Thus, the long-term effects of testosterone on body anthropometry are not known.
WHAT THIS STUDY ADDS:
Long-term testosterone treatment of hypogonadal men, up to 5 years duration, produced marked and significant decrease in body weight, waist circumference and body mass index.
Hypogonadism contributes to reduced muscle mass and increased adiposity.Testosterone treatment ameliorates loss of muscle mass and reduces fat accumulation associated with hypogonadism. In this study, we evaluated the long-term effects of normalizing testosterone (T) levels in hypogonadal men on anthropometric parameters. Open-label, single-center, cumulative, prospective registry study of 261 men (32-84 years, mean 59.5 ± 8.4 years, with T levels ≤12 nmol L-1 [mean: 7.7 ± 2.1]).
Among the 261 men on T treatment, we followed up on 260 men for at least 2 years, 237 for 3 years, 195 for 4 years and 163 for at least 5 years. Subjects received parenteral T undecanoate 1000 mg every 12 weeks after an initial interval of 6 weeks. Body weight (BW), waist circumference (WC) and body mass index (BMI) were measured at baseline and yearly after treatment with T. BW decreased from 100.1 ± 14.0 kg to 92.5 ± 11.2 kg and WC was reduced from 107.7 ± 10.0 cm to 99.0 ± 9.1 cm. BMI declined from 31.7 ± 4.4 m kg-2 to 29.4 ± 3.4 m kg-2. All parameters examined were statistically significant vs. baseline and vs. the previous year over 5 years, indicating a continuous weight loss (WL) over the full observation period. The mean per cent WL was 3.2 ± 0.3% after 1 year, 5.6 ± 0.3%, after 2 years, 7.5 ± 0.3% after 3 years, 9.1 ± 0.3% after 4 years and 10.5 ± 0.4% after 5 years. The data obtained from this uncontrolled, observational, registry study suggest that raising serum T to normal physiological levels in hypogonadal men produces consistent loss in BW, WC and BMI. These marked improvements were progressive over the 5 years of the study.
Got Fat? Pass the Testosterone Please!
Consider this: how many times have you read or heard that there is no such thing as a “cutting” steroid? For years the generally accepted doctrine was that steroids do not reduce adipose tissue but rather can decrease water retention and inhibit estrogen’s actions thus producing the “hard” look. Furthermore, the consensus seems to be that while testosterone and related anabolic substances certainly aid the dieting bodybuilder it is only via the androgen’s anti-proteolytic abilities, thus sparing muscle tissue while in a hypocaloric state, in addition to the aforementioned “drying out” effect.
In short – this is hogwash!! As this article will validate, an abundance of clinical research and peer-reviewed data strongly supports testosterone’s (T) fat reducing actions and its preventative impact on adipocyte generation (1,2,3). Therefore, T acts both in the breakdown of existing fat tissue and to hinder pre-adipocytes from maturing. CCAAT enhancer-binding proteins (CEBPs) are vital contributors at several stages of adipogenesis – or fat cell formation – from early uptake and accumulation of lipids to the differentiation, proliferation, and terminal production of the adipocyte.
CEBPs exist in an alpha, sigma, and beta form. The beta and sigma form promote the transcription of PPARy2, which is arguably the most important factor in fat cell formation. CEBP possesses intrinsic pro-adipogenic activity independent of PPARy2. Specifically, the alpha and sigma isoform of CEBP interact with promoter regions of DNA involved in adipose development (5). PPARy2, stimulated by the CEBP isoforms, is capable of the same. This leads to a viscous cycle beginning with CEBP activity. DNA promotion of said adipogenic proteins follows, including PPARy2, which itself then activates the same lipid-related genes as the CCAAT enhancer-binding proteins.
Testosterone has been shown to reduce all three forms of CEBP’s, which, predictably, curtails PPARy2 functions (5, 6, 7, 17, 23). Evidence exists as well that dihydrotestosterone shares T’s anti-CEBP characteristics (6, 23). Like muscle tissue, fat cells express anabolic receptors, which bind to respective androgens and initiate inhibitory effects on CEBP’s. Visceral Adipose Tissue (VAT) is more sensitive to T than subcutaneous fat (SC). This is most likely explained by the fact that AR’s occur in greater number in VAT versus SC tissue (2, 3, 4). T’s strong effect on VAT coincides with the observed reduction in abdominal fat in hypogonadal men placed on testosterone therapy. Furthermore, T’s antiadiogenic and lipolytic actions are more predominant in VAT (1, 2). Pre-adipocytes, located in VAT, show reduced activity of the CEBP’s when exposed to testosterone (5, 6). A novel observational study followed 511 males aged 30-79, over a 12-year period charting the effects of serum androstenedione, testosterone, and SHBG and their relation to VAT.
Hormone analysis was conducted before and after this period and concluded that below normal androstenedione and testosterone show strong correlation with abdominal adipose tissue. (8). Peroxisomal proliferator-activated receptor gamma 2 is another protein that functions to increase the differentiation of adipocytes. Treatment with either testosterone or DHT reduces PPARg2 providing another means whereby androgens discourage the differentiation stage in adipogenesis (22) The metabolic alterations experienced in VAT due to testosterone are of significance with both negative and positive ramifications. Lipid uptake is inhibited in VAT mainly due to a decrease in lipoprotein-lipase activity, and a reduction in serum insulin and glucose levels (9). It should be noted that lipid uptake is enhanced by elevated hyperglycemia/hyperinsulinemia, primarily in VAT (10). So the decrease in VAT noted with a reduction of glucose/insulin levels – via testosterone elevation – should make sense.
The ability of testosterone to stimulate fatty acid release from VAT can be problematic, though. Feeding directly into the liver via the portal vein, these fatty acids have the potential to impair both insulin sensitivity and function, induce hyperglycemia, and increase LDL levels. Ultimately this can lead to hyperinsulinemia, hyperglycemia, hyperlipidemia, and hypertension, vascular resistance, all of which are consistent with the pathology of “Metabolic Syndrome X” (11). In addition, cortisol, originating in VAT, is also released and fed to the liver via the portal vein. This exacerbates fat cell maturity as cortisol increases the differentiation of pre-adipocytes into the adult forms (13). It would seem logical then to suggest a cause and effect relationship between free fatty acid release from VAT by testosterone and the development the conditions of Metabolic Syndrome X. This may be a moot point though as testosterone treatment has been associated with an actual reduction in both total cholesterol and LDL levels. Previous studies indicate that testosterone – especially its chemically altered versions – can lower HDL levels deemed the “good cholesterol”. This has not been demonstrated conclusively, as results of other research efforts imply that T is neutral in regards to HDL, neither causing a significant increase or decrease (12, 16). In relation to the cardiovascular disorders, normal or slightly enhanced T levels might exert a protective effect. Pro-inflammatory cytokines TNF , IL-1ß, and IL-6 in human macrophages, are positively associated with congestive heart failure and atherosclerosis.
Not only has testosterone been shown to reduce the elevations in these cytokines but also up regulates anti-inflammatory cytokines such as IL-10, leading to an improvement in improving overall cardiovascular function. (12, 14, 15) This decreases susceptibility to congestive heart failure and vascular disease also due in part to T’s vasodilating characteristics.In relation to existing fat tissue, T levels are inversely associated with lipoprotein lipase, an enzyme involved in the breakdown of triglycerides and recycling of the resulting fatty acids and eventual production a mature lipid-rich adipocyte. Testosterone also impairs overall lipid uptake into fat cells, hindering hypertrophy of existing fat cells. T is positively correlated with beta receptor number catecholamine sensitivity, thus heightening thermogenesis (18, 19, 20). The thermogenic process involves a G-protein activation and a downstream production of lipolytic enzymes and proteins. Both DHT and Testosterone amplifies adenylate cyclase activity, an enzyme vital to beta receptor stimulated lipolysis (21). Moreover, hormone-sensitive lipase (HSL) initiates the breakdown of stored tryiglycerides into fatty acids, which can be oxidized and thus “burned” in either mitotochondria and/or peroxisomes. T not only elevates HSL but also Protein Kinase A, an enzyme that functions in the G-protein stimulated lipolysis (18, 20) By jacking up these lipolytic enzymes and proteins, beta oxidation occurs.
Androgens also increase other such enzymes including carboxylesterase 3, acetyl-coenzyme A acyltransferase 1, 3-ketoacyl-CoA thiolase B and enoyl-coenzyme A hydratase/3-hydroxyacyl coenzyme A dehydrogenase (2, 24). Enzymes vital to lipogenesis, conversely, show a consistent negative correlation with both T and DHT. Malic enzyme, glyceraldehyde dehydrogenase (GDH), and fatty acid elongation factors – all capable of pro-lipogenic effects, are inhibited by DHT and T (2, 24) It is of great importance, though, to understand that the T’s positive benefits espoused in this article DO NOT apply to females. In fact, typically, the majority of natural male androgens, such as testosterone and DHT, exert converse or opposing actions – often negative – in females compared to males. Furthermore, the studies and research cited herein refer to inherent activity of endogenous T and DHT or hormone replacement therapy (HRT). These intrinsic natural androgens and their actions, thus, do not extend to chemical altered androgens or anabolic steroids. This does not necessarily imply that AAS do not have the potential to mimic endogenous male hormone.
This just indicates that only research reviews examining naturally produced androgens were considered herein. If truth be told, a follow-up article delving even deeper into functions of T on obesity would certainly be justified. It also could review effects of both testosterone in females and chemically altered androgens. This article provides a fairly brief review of what is actually an impressive and growing body of evidence supporting Testosterone and other androgens as potent lipolytic and anti-adipogenic hormones. In fact, while the author had reviewed relevant literature beforehand describing the interactions of androgens and adipose, the additional dissection of this topic revealed a much greater than anticipated number of mechanisms and actions of natural androgens. Testosterone has not really received the respect and appreciation it deserves for its wide range of health benefits. Recently, though, with the ever-increasing administration of HRT to males and evidence confirming its potential benefits, the negative connotation and exaggerated drawbacks of such treatment is dying out. In its place exists a wealth of legitimate research claims and an increased priority placed on unbiased future studies.
-------------------------------------------------------------------------------------------------------------------------------------------------------------
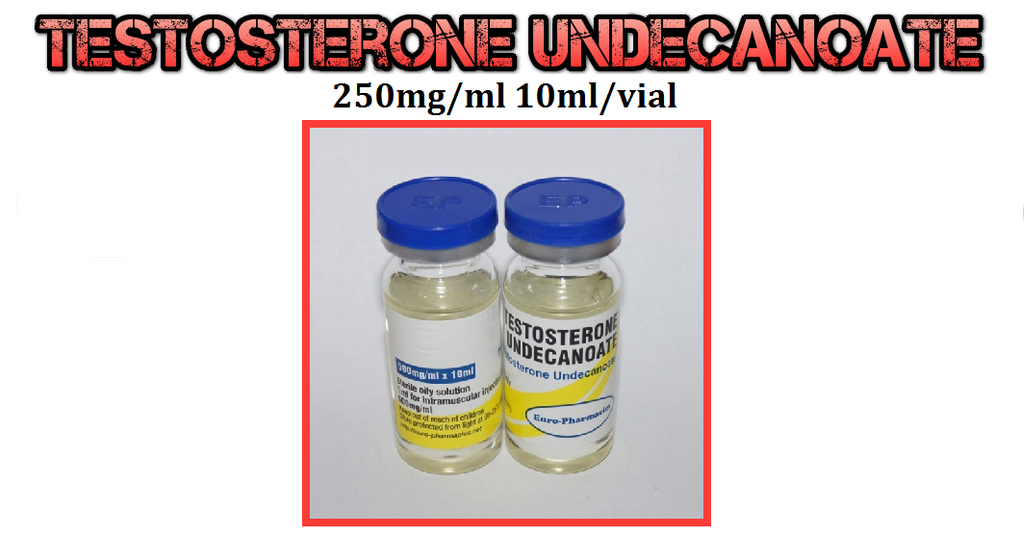
The best Testosterone for the sole purpose of fat lose
Here we have Testosterone Undecanoate
"Here at PSL we have all of your HRT/TRT needs covered, with a vast amount of Testosterone ester hormones"
http://www.puritysourcelabs.com/inje...mlvial-ep.html
This shrouded compound is truly a contender in the compound arsenal with great reason, as TU can be utilized in a cruise,HRT or even a cycle stack where multi compounds are implemented.
Injections scheduling can be decreased with your testosterone in take, at the same time freeing up more injection sites/ for more volume with other compounds, at the same time keeping you test levels up to par with injections as often as every 10-16 days (Half life of Test Undecanoate is 16 days)
Dosages may range from 250-500 weekly, or 500-1000, and the injections can be spaced out up until 10-14 days (or more)
TU is used widely in many clinics world wide to treat men with hypogonadism, and HRT therapy..
The amazing benefits from this compound yields less injections,a much more stable blood environment, less fluctuations, with the convenience of freeing up injection sites for other AAS compounds that may be needed in ones protocol..
This Test ester is very diverse, serving multi purposes!
Study below concerning TRT treatment with TU
__________________________________________________ ___________________
Treatment of male hypogonadism with testosterone undecanoate injected at extended intervals of 12 weeks: a phase II study.
Abstract
This paper reports the result of an open-label, non-randomized clinical trial investigating the efficacy and safety of an injectable preparation of testosterone undecanoate (TU) dissolved in castor oil and given over a 3.2-year period. In a previous study we demonstrated that injections of TU every 6 weeks resulted in satisfactory substitution but a tendency toward testosterone accumulation. Here we investigate prolonged TU treatment at extended injection intervals in 7 hypogonadal men. Injections were given at gradually increasing intervals between the fifth and 10th injection, and from then on every 12 weeks. Steady state kinetics were obtained after the 13th injection. Well-being, sexual activity, clinical chemistry, prostate volume, and prostate-specific antigen (PSA) and serum hormone levels were monitored. Patients were clinically well-adjusted throughout the study. Before the next injection, testosterone, dihydrotestosterone, and estradiol levels were mostly within the normal range and showed a tendency to decrease with increasing injection intervals. Body weight, hemoglobin, serum lipids, PSA, and prostate volume did not change significantly during the 3.2 years of treatment. PSA levels were always within the normal limit. Maximal testosterone levels during steady state kinetics were measured after 1 week with 32.0 +/- 11.7 nmol/L (mean +/- SD). Before the last injection, mean testosterone concentrations were 12.6 +/- 3.7 nmol/L. Compared with conventional testosterone enanthate or cypionate treatment requiring injection intervals of 2-3 weeks and resulting in supraphysiological serum testosterone levels, injections of TU at intervals of up to 3 months offer an excellent alternative for substitution therapy of male hypogonadism.
American Society of Andrology
Treatment of Male Hypogonadism With Testosterone
Undecanoate Injected at Extended Intervals of 12 Weeks: A
Phase II Study
SIGRID VON ECKARDSTEIN AND EBERHARD NIESCHLAG
From the Institute of Reproductive Medicine of the University, D-48129 Munster, Germany
Testosterone preparations have been in clinical use for
substitution of male hypogonadism for more than a
half century. However, only within recent years has the
choice of different delivery forms increased. The newly
developed transdermal preparations having short half-
lives are predominantly tailored for therapy of senescent
men (Nieschlag, 1998). For substitution of younger hy-
pogonadal men and for hormonal male contraception,
long-acting substances are more desirable. Therefore, the
development and first clinical uses of injectable testoster-
one buciclate (Behre et al, 1995) and testosterone unde-
canoate (TU; Partsch et al, 1995; Zhang et al, 1998; Behre
et al, 1999a) attracted much attention.
Six-week injections of 1000 mg TU in 4 mL castor oil
resulted in well-maintained androgen-dependent functions
without serious side effects (Nieschlag et al, 1999). How-
ever, after 4 injections, a tendency toward a gradual in-
crease in testosterone levels was observed, suggesting that
prolongation of application intervals should be possible.
In the present paper we report the results of continued
substitution therapy with TU in 7 hypogonadal men, and
explore the efficacy and safety of injection intervals of
up to 12 weeks, for a total period of 3.2 years.
Materials and Methods
Patients
Seven men with primary or secondary hypogonadism aged 20
to 57 years, who had already participated in the first trial with
6-week injections of TU agreed to receive continuing treatment.
Inclusion and exclusion criteria to enroll patients for TU treat-
ment have been described previously (Nieschlag et al, 1999).
Prolongation of the initial study protocol was approved by the
ethics committee of the university and the State Medical Board,
Munster. Written informed consent was obtained from subjects.
Rules for clinical studies as provided by the Declaration of Hel-
sinki and the standards of good clinical practice were followed.
Five patients entering the follow-up phase had primary hy-
pogonadism, and 2 had secondary hypogonadism. The limit of
serum testosterone for establishing the diagnosis of hypogonad-
ism in our institute is 12 nmol/L. Diagnosis and the previous
mode of substitution are given in Table 1. Two men had previ-
ously participated in the initial study comparing the pharmaco-
kinetics of TU dissolved in either tea seed (‘‘Chinese prepara-
tion’’) or castor oil (Behre et al, 1999a)
May/June 2002
Table 1.
Clinical characteristics of patients entering the follow-up phase of substitution therapy with 1000 mg TU
Patient Diagnosis Age (y)† Treatment (before TU)†
1 Bilateral orchidectomy due to seminoma 37 TE 250 mg/4 weeks
2 Bilateral testicular atrophy due to cryptorchidism 19 None
3 Bilateral orchidectomy due to seminoma age 49 TE 250 mg/3 to 4 weeks
4 Bilateral orchidectomy due to seminoma age 37 None
5 Bilateral orchidectomy due to seminoma age 57 TE 250 mg/3 to 4 weeks
6 Bilateral orchidectomy due to seminoma age 31 TE 250 mg/2 to 4 weeks
7 Hypogonadotropic hypogonadism (ectopic neurohypophysis age 29 TE 250 mg/4 weeksJournal of Andrology
* Patients who had already participated in the study on comparative pharmacokinetics with TU in castor oil or tea seed oil.
† Age and previous treatment modalities refer to the date before the first TU application. TE indicates testosterone enanthate
Testosterone Preparation
TU was obtained from Jenapharm GmbH & Co. KG, Jena, Ger-
many. Each ampule contained 1000 mg TU dissolved in 4 ml
castor oil. Single injections were administered with the total vol-
ume at one site intramuscularly into the musculus gluteus med-
ius, taking care to perform injections slowly to avoid pain.
Study Design
The study was a clinical, open label, nonrandomized trial.
Screening examinations had been completed before the first in-
jection with TU as described previously (Nieschlag et al, 1999).
Before the first TU application, all men under current treatment
completed a washout phase of at least 4 weeks. An overview of
studies evaluating TU, including the current design, is given in
Table 2. Before entering the follow-up phase, all patients under-
went another complete physical and genital investigation and
assessment of clinical chemistry, hematology, and lipids, as well
as sonography of testes and prostate. Well-being and sexuality
were investigated by standardized questionnaires immediately
before and at half-time between TU injections. Before each ap-
plication, blood samples for measurements of hormones, sex
hormone binding globulin (SHBG), albumin, PSA, clinical
chemistry, lipidology, and hematology were obtained. Prostate
size was determined sonographically before every second injec-tion.
After 4 injections had been given at 6-week intervals, the
intervals were gradually extended between the 5th and 10th in-
jections. Intervals were extended by 1 to 2 weeks if serum tes-
tosterone levels were above 12 nmol/L before the next injection,
and if subjective impairment of well-being was absent. From the
10th injection onward, TU was applied every 12 weeks. After
the 13th application, steady state kinetics were obtained as evi-
denced by weekly determinations of testosterone serum concen-
trations for 12 weeks. Six weeks after the 18th application, the
study was finished with a detailed final investigation, including
a physical and genital examination, sonography of prostate and
scrotal contents, and all blood values that had been monitoredduring the study.
Hormone Assays
Analysis was performed from venous blood samples that were
centrifuged at 800
3g
for 10 minutes and then stored at
220
8C
until measurements were performed at the end of the study. Care
was taken so that samples of one subject were measured withinone assay.
Serum concentrations of follicle-stimulating hormone (FSH),
luteinizing hormone (LH), estradiol, SHBG, prolactin, and PSA
were analyzed by highly specific time-resolved immunofluoro-
metric assays (Autodelfia; Wallac, Freiburg, Germany). The low-
er detection limits were 0.12 IU/L and 0.25 IU/L for FSH and
LH, respectively; and 25 pmol/L, 6.3 nmol/L, and 0.5mg/L for
estradiol, SHBG, and PSA, respectively. The normal range in
our laboratory is 1–7 IU/L and 2–10 IU/L for FSH and LH,
respectively, and 11–71 nmol/L for SHBG. The upper limits of
normal for estradiol and PSA are 250 pmol/L and 4m
g/L,
respectively. The intraassay and interassay coefficients of variation
were 0.5 and 1.9 for FSH, 1.7 and 2.2 for LH, 1.9 and 5.0 for
estradiol, 1.0 and 7.2 for SHBG, and 3.4 and 4.9 for PSA. Serum
testosterone was measured by an enzyme-linked immunosorbent
assay (Biocam Immunosystems; DRG Instruments, Marburg,
Germany). The lower limit of normal is 12 nmol/L. Dihydrotes-
tosterone (DHT) was analyzed by radioimmunoassay (DSL
9600; Diagnostic System Laboratories, Sinsheim, Germany). In-
traassay and interassay coefficients of variation for testosterone
and DHT were 3.4 and 5.6, and 4.8 and 9.2, respectively. Free-
testosterone was calculated using the formula suggested by Ver-
meulen et al (1999)
Eckardstein and Nieschlag ·
Injectable Testosterone Undecanoate
Clinical Chemistry, Hematology, and Lipids
Biochemical and hematological parameters were determined at
the Institute of Laboratory Medicine, University of Mu
̈ nster us-
ing, standard techniques. Quality control was performed accord-
ing to the standards provided by the German Society of Clinical
Chemistry.
Evaluation of Well-Being and Sexuality
During treatment patients were asked to complete standardized
questionnaires to assess mood and sexual performance. Com-
pleted questionnaires were obtained before injections and at the
halfway point of the respective injection interval.
Evaluation of Prostate
Prostate volume was monitored by transrectal ultrasound using
a 7.5 MHz probe (The Panther; B&K Medical, Norderstedt, Ger-
many). Prostate examinations included planimetric determination
of volume (Behre et al, 2000) and assessment of sonographic
texture.
Statistics
Statistical analysis was performed using the SPSS statistical
package for Windows (version 10.0). All variables were checked
for normal distribution by the Kolmogorov-Smirnov one-sample
test for goodness-of-fit. Descriptive statistics are given as either
means
6
SD or median, and the 2.5 to 97.5 percentiles. For
analysis of variance (ANOVA) over time, one-way analysis of
variance was calculated, which was followed by the Dunnett
post-hoc test for intergroup comparison if an overall level of
significance of P
.05 was reached. When necessary, analysis
was performed after logarithmic transformation of data.
Results
General Effects, Well-Being, and Sexual Function
During TU applications, patients reported stable values
for all parameters of well-being and sexual function
(numbers of erections and ejaculations per week and sat-
isfaction with sex life). At the end of the injection inter-
val, when questionnaires were compared with those at
half-time, no statistically significant differences werefound.
Injections were well tolerated by all men except one,
who requested extremely slow injections to avoid discom-
fort. No local side effects or impaired well-being oc-
curred, except for one occasion when, during prostate so-
nography, a patient had short-term circulatory problems
after the injection. One patient complained initially of
mild acne within 2 weeks following injection. However,
these problems disappeared during the 12-week intervals.
In addition, the same patient developed slight gyneco-
mastia during the first part of the study (6 weekly injec-
tions), which remained unchanged during the follow-up
period.
General adverse events related to the treatment were
not observed. One patient experienced an episode of her-
pes zoster, which required antiviral therapy after severe
psychological trauma.
Body Weight
During TU applications, body weight increased slightly
from 83.5
6
9.5 kg to 85.7
6
9.1 kg without reaching
the level for statistical significance. Compared with the
baseline, the maximum mean body weight was observed
at the end of the study period.
Testosterone and Free Testosterone
Testosterone serum levels and calculated free testosterone
levels obtained before injections are shown in Figure 1.
During the 6-week injection interval, testosterone levels
increased initially from 5.2
6
3.1 nmol/L to 23.8
6
7.8
nmol/L after patients had received 4 injections in 6 weeks.
With extended injection intervals, preapplication testos-
terone levels decreased and were just at the lower limit
of normal, with 12.6
6
3.7 nmol/L before the last injec-
tion. A comparable pattern was observed for calculated
free testosterone levels, which rose to 573
6
202 pmol/
L after the 6-week period, and then returned to the lower
limit of normal (291
6
93 pmol/L) after 8 injections had
been performed at 12-week intervals.
Maximum steady state kinetics for levels of testoster-
one and free testosterone were reached after 1 week. The
mean maximum concentration for testosterone was 32
nmol/L, ranging from a minimum of 15.6 to a maximum
of 44.3 nmol/L. A comparable pattern was observed for
free testosterone levels, with a mean of 787 pmol/L (Table
3). Initial kinetics obtained in 14 subjects after the first
injection of TU and steady state kinetics in the current
trial are shown in Figure 2.
Estradiol and DHT
DHT and estradiol concentrations essentially followed the
pattern of that for testosterone and free testosterone. Dur-
ing the short injection intervals, DHT levels occasionally
exceeded the upper normal limit but returned to the lower
limit of normal after 5 injections over 12 weeks had been
applied (Figure 1). Estradiol levels always stayed within
normal limits.
LH and FSH
LH and FSH values decreased significantly during the
study, from initial values of 18.7
7.1 IU/L (LH) and 30.5,
27.3 IU/L (FSH) to 0.4,
0.8 IU/L (LH) and 1.5,
2.9 IU/L (FSH) after 24 weeks. Before the last injec-
tion, LH values of 3.0,
5.0 IU/L and FSH values of 7.7,
13.9 IU/L were measured

























































 Reply With Quote
Reply With Quote
Bookmarks